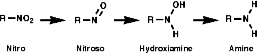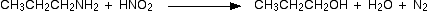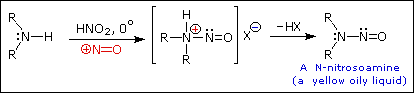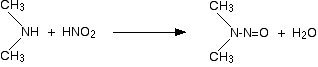1. Introduction
Some basic knowledge to get in Organic Chemistry.
- Introduction Part 1
- Introduction Part 2
2. Alkane
The easiest molecule to start.
3. Alkene
Just like Alkane, only some double bonds.
4. Aromatic
When carbon gets into cycle.
- Examples of Miscellaneous Reactions of Aromatic Compounds 1
- Examples of Miscellaneous Reactions of Aromatic Compounds 2
5. Carbonyl Group
Oxygen has a double bond with Carbon.
- Aldehyde and Ketone 1
- Aldehyde and Ketone 2
6. Carboxylix Acid
The well-known organic acid.
7. Carboxylic Acid Derivatives
Some functional groups similar to carboxylic acid.
- Ester and Amide 1
- Ester and Amide 2
8. Amine
Let's try out Nitrogen.
Thursday, February 17, 2011
Tuesday, February 15, 2011
Organic Chemistry : Amine
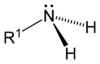
Amine is a Nitrogen functional group. (Excluding Amide, which we have known as a more specific functional group.) It can be -NH2 or, possibly, H in -NH2 can also be replaced with other groups such as -NHCH2CH3.
IUPAC Names
- Find the longest carbon chain containing the amine group.
- The position of any group attached to Nitrogen atom is denoted as N. (We need to ignore alphabet arrangement for N position because N position must be arranged at the first if exists.)
- Then, the name is formed by changing the -e of the alkane one to -amine.
- The position of Nitrogen attached to Carbon is indicated by number before -amine.
- Example : CH3CH2CH(NHCH2CH3)CH(CH2CH3)CH2CH2CH3 = N,4-diethyl-3-heptanamine.
Fun Facts : a well-known illegal drug, amphetamine, is actually 1-phenyl-2-propanamine.
Physical Properties
Boiling Point
With N-C bonding, it has higher boiling point than other unpolar organic substances, but still lower than other O-C-bonding polar ones such as Carboxylic acid or Alcoho.
Solubility
With its polar, it is soluble with water, except only aromatic amine. As usual, the bigger of molecule, the lower solubility.
Base of Amine
Since Nitrogen has 5 electrons and 3 of them are used for 3 single bonds, there are 2 of them left. Those 2 can be used to donate to Hydrogen ion (Proton.) Thus, Amine is base.
Reaction
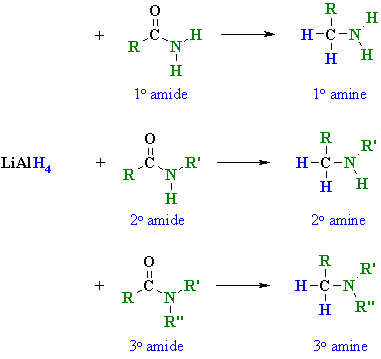
1. Amide can be yield by reduction of Nitro.
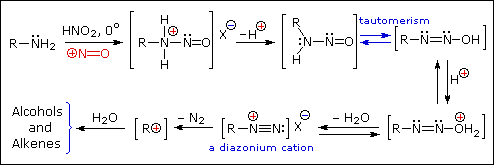
2. Reaction with Nitrous acid.
- Primary
- Secondary
Reference : BCC M.5 Chemistry Book.
http://en.wikipedia.org/wiki/Amine
http://www2.chemistry.msu.edu
http://www.chemguide.co.uk
http://www.pharmgkb.org
| Primary amine | Secondary amine | Tertiary amine |
|---|---|---|
 |  |  |
IUPAC Names
- Find the longest carbon chain containing the amine group.
- The position of any group attached to Nitrogen atom is denoted as N. (We need to ignore alphabet arrangement for N position because N position must be arranged at the first if exists.)
- Then, the name is formed by changing the -e of the alkane one to -amine.
- The position of Nitrogen attached to Carbon is indicated by number before -amine.
- Example : CH3CH2CH(NHCH2CH3)CH(CH2CH3)CH2CH2CH3 = N,4-diethyl-3-heptanamine.
Fun Facts : a well-known illegal drug, amphetamine, is actually 1-phenyl-2-propanamine.
Physical Properties
Boiling Point
With N-C bonding, it has higher boiling point than other unpolar organic substances, but still lower than other O-C-bonding polar ones such as Carboxylic acid or Alcoho.
Solubility
With its polar, it is soluble with water, except only aromatic amine. As usual, the bigger of molecule, the lower solubility.
Base of Amine
Since Nitrogen has 5 electrons and 3 of them are used for 3 single bonds, there are 2 of them left. Those 2 can be used to donate to Hydrogen ion (Proton.) Thus, Amine is base.
Reaction
1. Amide can be yield by reduction of Nitro.
2. Reaction with Nitrous acid.
- Primary
Example :
- Secondary
Example :
- Tertiary
You will get nothing! (Just a weak base reacting with weak acid.)
Reference : BCC M.5 Chemistry Book.
http://en.wikipedia.org/wiki/Amine
http://www2.chemistry.msu.edu
http://www.chemguide.co.uk
http://www.pharmgkb.org
Organic Chemistry : Ester and Amide 2
Ester Reaction
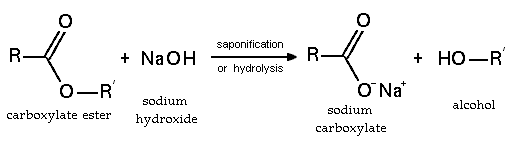
1. Ester can be yield as below. Do it backward called Hydrolysis
2. Reduction
3. Hoffmann Rearrangement
Reference : BCC M.5 Chemistry Book.
http://en.wikipedia.org/wiki/Ester
http://en.wikipedia.org/wiki/Amide
http://image.wistatutor.com
1. Ester can be yield as below. Do it backward called Hydrolysis
2. Saponification
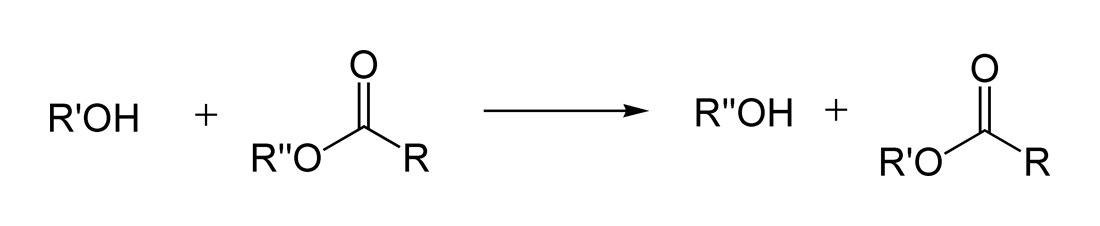
3. Transesterification
4. Reduction
Amide Reaction
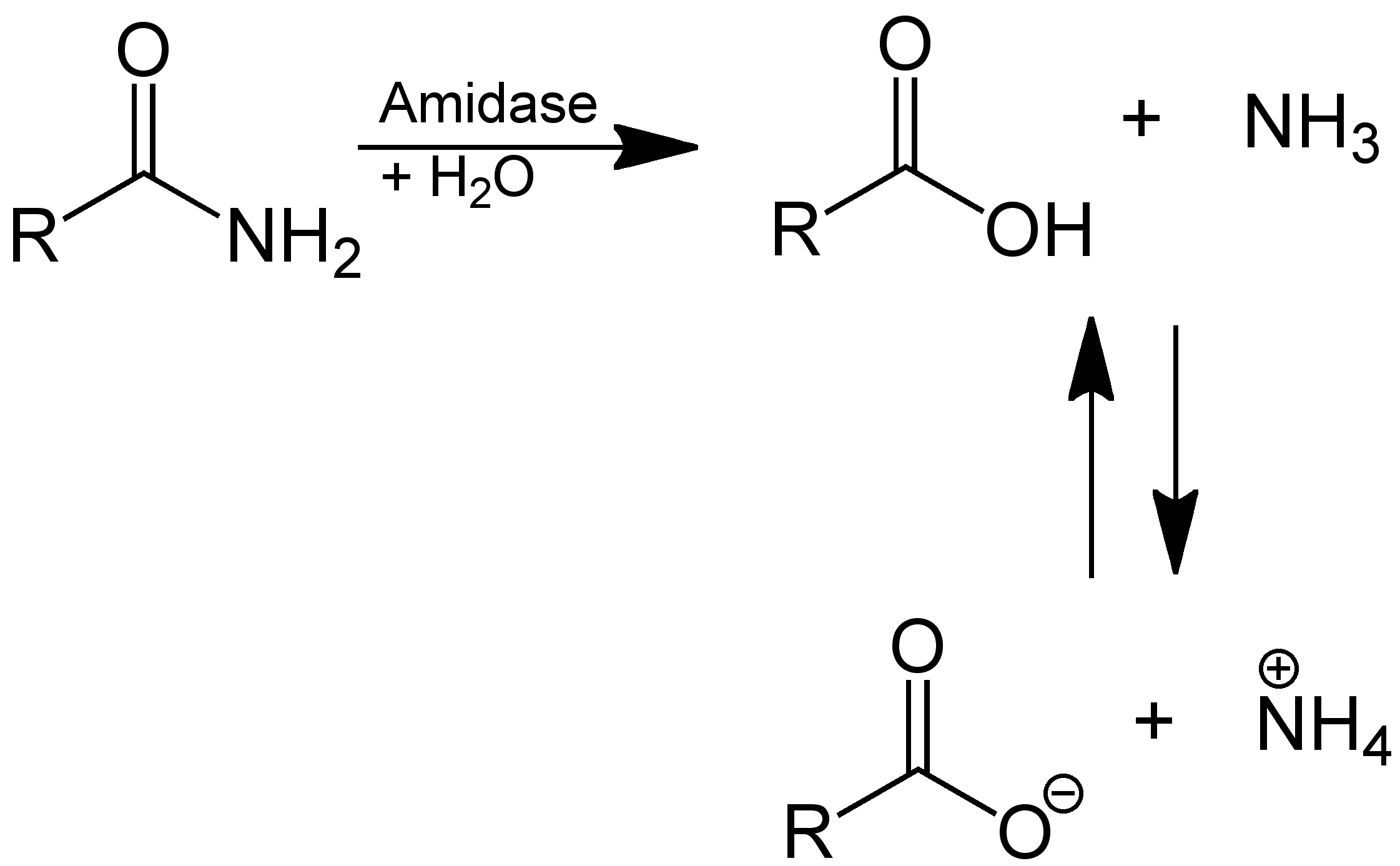
1. Hydrolysis
3. Hoffmann Rearrangement
http://en.wikipedia.org/wiki/Ester
http://en.wikipedia.org/wiki/Amide
http://image.wistatutor.com
Organic Chemistry : Ester and Amide 1
From previous group, -COOH, sometimes we have some slightly different organic substance, just replace H in -COOH into others. For example, -COOCH3 , -COONH2. These are called Carboxylic Acid Derivatives. Here, we will study only two big ones, Ester and Amide.
- Ester : Replace H in -COOH with some other group such as -COOCH2CH3.
- Amide : Replace H in -COOH with N such as -COONHCH2CH3.
IUPAC Names
Ester
- Find the longest carbon chain containing the ester group, where the position of the carbonyl carbon must be the first.
- Call the secondary group that replaces the H in -COOH, then call the other side. (the carbon chain side)
- Then, the name is formed by changing the -e of the alkane one to -oate.
- Example : CH3CH2CH2CH2-COOCH2CH3 = ethylpentanoate
Amide
- Find the longest carbon chain containing the amide group, where the position of the amide carbon must be the first.
- Call the secondary group (-NRR') that replaces the H in -COOH, then call the other side. (the carbon chain side)
- The position of the Nitrogen atom is denoted as N. (We need to ignore alphabet arrangement for N position because N position must be arranged at the first if exists.)
- Then, the name is formed by changing the -e of the alkane one to -oate.
- Example : CH3CH3CH2CH(CH2CH3)CH2-COONHCH(CH3)2 = N-1-methylethyl-3-ethylhexanamide
Physical Properties
Boiling Points
Amide is the organic substance with the highest boiling point because of dimers (same as carboxylic acid, but stronger.) Ester's boiling point is really low, since the polar around carbonyl group is pretty weak compared to Aldehyde, Ketone, or Carboxylic acid.
Reference : BCC M.5 Chemistry Book.
http://en.wikipedia.org/wiki/Ester
http://en.wikipedia.org/wiki/Amide
http://image.wistatutor.com
- Ester : Replace H in -COOH with some other group such as -COOCH2CH3.
- Amide : Replace H in -COOH with N such as -COONHCH2CH3.
IUPAC Names
Ester
- Find the longest carbon chain containing the ester group, where the position of the carbonyl carbon must be the first.
- Call the secondary group that replaces the H in -COOH, then call the other side. (the carbon chain side)
- Then, the name is formed by changing the -e of the alkane one to -oate.
- Example : CH3CH2CH2CH2-COOCH2CH3 = ethylpentanoate
Amide
- Find the longest carbon chain containing the amide group, where the position of the amide carbon must be the first.
- Call the secondary group (-NRR') that replaces the H in -COOH, then call the other side. (the carbon chain side)
- The position of the Nitrogen atom is denoted as N. (We need to ignore alphabet arrangement for N position because N position must be arranged at the first if exists.)
- Then, the name is formed by changing the -e of the alkane one to -oate.
- Example : CH3CH3CH2CH(CH2CH3)CH2-COONHCH(CH3)2 = N-1-methylethyl-3-ethylhexanamide
Physical Properties
Boiling Points
Amide is the organic substance with the highest boiling point because of dimers (same as carboxylic acid, but stronger.) Ester's boiling point is really low, since the polar around carbonyl group is pretty weak compared to Aldehyde, Ketone, or Carboxylic acid.
Solubility
Carboxylic Acid Derivatives are mostly the same as old carboxylic acids. So small carboxylic acids derivatives are usually soluble.Reference : BCC M.5 Chemistry Book.
http://en.wikipedia.org/wiki/Ester
http://en.wikipedia.org/wiki/Amide
http://image.wistatutor.com
Organic Chemistry : Carboxylix Acid
Carboxylic acids are organic substance with carboxyl group; R-COOH, where R is some monovalent functional group. (lower priority groups.) A carboxyl group (or carboxy) is a functional group consisting of a carbonyl (RR'C=O) and a hydroxyl (R-O-H) so it has the formula -C(=O)OH, which is usually written as -COOH.
- Then, the name is formed by changing the -e of the alkane one to -oic acid.
- The position of the carboxy group is always the first.
- Example : CH(CH3)2COOH = 2-methylpropanoic acid.
IUPAC Names
- Find the longest carbon chain containing the carboxy group.- Then, the name is formed by changing the -e of the alkane one to -oic acid.
- The position of the carboxy group is always the first.
- Example : CH(CH3)2COOH = 2-methylpropanoic acid.
Physical properties
Solubility
Carboxylic acids are polar because there are both hydrogen-bond acceptors (the carbonyl) and hydrogen-bond donors (the hydroxyl). So they can do hydrogen bonding to water. However, only small carboxylic acids (1 to 5 carbons) are soluble.Boiling points
Carboxylic acids have highboiling points because of their two hydrogen bonds per pair of molecules (called dimers.)Acidity
Carboxylic acids are weak acids. Here are some examples :| Carboxylic Acids | pKa |
|---|---|
| Formic acid (HCO2H) | 3.77 |
| Acetic acid (CH3COOH) | 4.76 |
| Chloroacetic acid (CH2ClCO2H) | 2.86 |
| Dichloroacetic acid (CHCl2CO2H) | 1.29 |
| Trichloroacetic acid (CCl3CO2H) | 0.65 |
| Trifluoroacetic acid (CF3CO2H) | 0.5 |
| Oxalic acid (HO2CCO2H) | 1.27 |
| Benzoic acid (C6H5CO2H) | 4.2 |
Reactions
We can do reduction.
And we can also use Carboxylic acid to have other organic function groups.
Reference : BCC M.5 Chemistry Book.
http://en.wikipedia.org/wiki/Carboxylic_acid
http://tutorvista.com
http://www.bmrb.wisc.edu
Organic Chemistry : Aldehyde and Ketone 2
Now, let's move on to the reactions they can have.
1. Oxidation
Can be used to know whether substance is Aldehyde or Ketone. Note that Aldehyde is always easier to do reactions.
- Tollen's Reagent >> give Ag as positive test, this is why it can be called Silvor Mirror Test
- Benedict' Reagent >> give Cu2O, a yellow, orange, or red dregs.
- Fehling's Reagent >> same as Benedict's
2. Reduction
3. Clemensen Reduction
1. Oxidation
Can be used to know whether substance is Aldehyde or Ketone. Note that Aldehyde is always easier to do reactions.
- Tollen's Reagent >> give Ag as positive test, this is why it can be called Silvor Mirror Test
- Benedict' Reagent >> give Cu2O, a yellow, orange, or red dregs.
- Fehling's Reagent >> same as Benedict's
2. Reduction
3. Clemensen Reduction
Reference : BCC M.5 Chemistry Book.
http://en.wikipedia.org/wiki/Aldehyde
http://en.wikipedia.org/wiki/Ketone
Wednesday, February 9, 2011
Organic Chemistry : Aldehyde and Ketone 1
We have studied organic substances that consist of only Carbons and Hydrogens. (All we have studied is the difference when the bondings and structures are different.) From now, we are going to add a new one; Oxygen.
In this, we will consider Oxygen that has a double bond to Carbon only (called Carbonyl group), which can be classified as Ketone or Aldehyde , depending on the Carbon Oxygen is bonding to as below:
Note that "R" stands for any alkyl.
IUPAC Names
Aldehydes
- Find the longest carbon chain containing the aldehyde group. Carbon with aldehyde group is always numbered as first one.
- Then, the name is formed by changing the -e of the alkane one to -al.
- Examples : HCHO = methanal, CH3CH2CH2CHO = butanal.
Ketones
- Find the longest carbon chain containing the kerone group.
- Then, the name is formed by changing the -e of the alkane one to -one.
- The position of the carbonyl group is denoted by a number.
- Example : CH3-CO-CH3 = 2-propanone.
Physical Properties
The carbonyl group is polar as the EN of the oxygen is greater than carbon. So, oxygen has negeative polar while carbon has positive so that Aldehydes and Ketones can do hydrogen bonding with water. They are more soluble in water if they are not too big - more than 4 carbons. However, polar in Aldehydes and Ketones gives them somewhat higher boiling and melting points. (But still lower than some like alcohol or carboxylic acid.)
http://www.chm.bris.ac.uk
http://www.elmhurst.edu
In this, we will consider Oxygen that has a double bond to Carbon only (called Carbonyl group), which can be classified as Ketone or Aldehyde , depending on the Carbon Oxygen is bonding to as below:
Note that "R" stands for any alkyl.
IUPAC Names
Aldehydes
- Find the longest carbon chain containing the aldehyde group. Carbon with aldehyde group is always numbered as first one.
- Then, the name is formed by changing the -e of the alkane one to -al.
- Examples : HCHO = methanal, CH3CH2CH2CHO = butanal.
Ketones
- Find the longest carbon chain containing the kerone group.
- Then, the name is formed by changing the -e of the alkane one to -one.
- The position of the carbonyl group is denoted by a number.
- Example : CH3-CO-CH3 = 2-propanone.
Physical Properties
The carbonyl group is polar as the EN of the oxygen is greater than carbon. So, oxygen has negeative polar while carbon has positive so that Aldehydes and Ketones can do hydrogen bonding with water. They are more soluble in water if they are not too big - more than 4 carbons. However, polar in Aldehydes and Ketones gives them somewhat higher boiling and melting points. (But still lower than some like alcohol or carboxylic acid.)
Reference : BCC M.5 Chemistry Book.
http://en.wikipedia.org/wiki/Aldehyde
http://en.wikipedia.org/wiki/Ketone http://www.chm.bris.ac.uk
http://www.elmhurst.edu
Monday, February 7, 2011
Examples of Miscellaneous Reactions of Aromatic Compounds 2
2. Reaction of : Toluene + Nitric Acid
First, nice to meet you, Toluene.
Toluene (or toluol) is Benzene that has been replaced its Hydrogen with methyl (CH3.) For the process, we can do Friedel–Crafts reaction (but no details will be explained here.) Toluene's pictures :
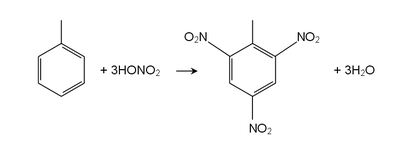
In the reaction to create the bomb, we need to replace another three nonneighboring Toluene's Hydrogen with NO2. In the first time, we use H2SO4 and HNO3 (Nitric Acid) to have mono-nitrotoluene or MNT from Toluene. Do Nitration again to MNT then we will get dinitrotoluene or DNT. Last, use dehydrate mixture of HNO3 and H2S2O7 with DNT and we will get trinitrotoluene or TNT.
Conclusion : replace Hydrogen with NO2 3 times.
And well, trinitrotoluene or TNT (C6H2(NO2)3CH3 as shown at the right side) is exactly the one we commonly hear as a bomb.
Refrerences :
http://en.wikipedia.org/wiki/Toluene
http://en.wikipedia.org/wiki/Trinitrotoluene
http://en.wikipedia.org/wiki/Mononitrotoluene
Let's make a bomb!!
First, nice to meet you, Toluene.
Toluene (or toluol) is Benzene that has been replaced its Hydrogen with methyl (CH3.) For the process, we can do Friedel–Crafts reaction (but no details will be explained here.) Toluene's pictures :
In the reaction to create the bomb, we need to replace another three nonneighboring Toluene's Hydrogen with NO2. In the first time, we use H2SO4 and HNO3 (Nitric Acid) to have mono-nitrotoluene or MNT from Toluene. Do Nitration again to MNT then we will get dinitrotoluene or DNT. Last, use dehydrate mixture of HNO3 and H2S2O7 with DNT and we will get trinitrotoluene or TNT.
Conclusion : replace Hydrogen with NO2 3 times.
And well, trinitrotoluene or TNT (C6H2(NO2)3CH3 as shown at the right side) is exactly the one we commonly hear as a bomb.
Refrerences :
http://en.wikipedia.org/wiki/Toluene
http://en.wikipedia.org/wiki/Trinitrotoluene
http://en.wikipedia.org/wiki/Mononitrotoluene
Examples of Miscellaneous Reactions of Aromatic Compounds 1
1. Polymerization of Styrene
We can have Polysterene (a kind of foam) by using continuously many Sterenes. Sterene, or Vinyl Benzene, is Benzene that has been replaced one of its Hydrogen with C2H2. So its formula is C6H5CH=CH2 as shown below.
Now, when we let Sterenes do a reaction at their C2H2 (because around their hexagon, they are aromatics, which are hard to do any reactions.) by destroying a pi bond in each of Sterenes in the free radical way so that unbonded electrons after the free radical will be used to be a sigma bond with other similar Sterenes.
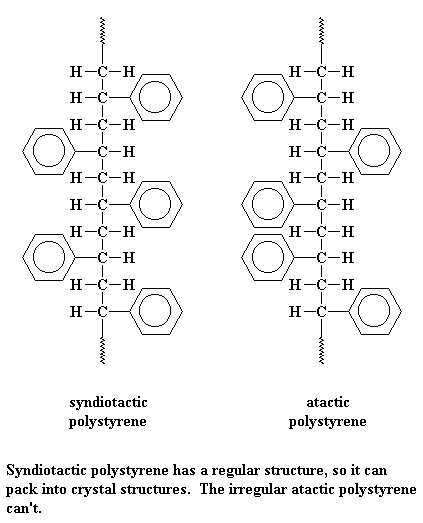
Bit of knowledge >>> Polysterene we get do not always need to have the aromatic groups (the hexagons you see) at the same side. For example: below are some of unneccessary ones.
References :
http://en.wikipedia.org/wiki/Styrene
http://en.wikipedia.org/wiki/Polystyrene
http://www.pslc.ws/mactest/styrene.htm
We can have Polysterene (a kind of foam) by using continuously many Sterenes. Sterene, or Vinyl Benzene, is Benzene that has been replaced one of its Hydrogen with C2H2. So its formula is C6H5CH=CH2 as shown below.
Now, when we let Sterenes do a reaction at their C2H2 (because around their hexagon, they are aromatics, which are hard to do any reactions.) by destroying a pi bond in each of Sterenes in the free radical way so that unbonded electrons after the free radical will be used to be a sigma bond with other similar Sterenes.
Bit of knowledge >>> Polysterene we get do not always need to have the aromatic groups (the hexagons you see) at the same side. For example: below are some of unneccessary ones.
References :
http://en.wikipedia.org/wiki/Styrene
http://en.wikipedia.org/wiki/Polystyrene
http://www.pslc.ws/mactest/styrene.htm
Subscribe to:
Posts (Atom)