| Primary amine | Secondary amine | Tertiary amine |
|---|---|---|
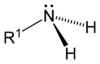 |  |  |
IUPAC Names
- Find the longest carbon chain containing the amine group.
- The position of any group attached to Nitrogen atom is denoted as N. (We need to ignore alphabet arrangement for N position because N position must be arranged at the first if exists.)
- Then, the name is formed by changing the -e of the alkane one to -amine.
- The position of Nitrogen attached to Carbon is indicated by number before -amine.
- Example : CH3CH2CH(NHCH2CH3)CH(CH2CH3)CH2CH2CH3 = N,4-diethyl-3-heptanamine.
Fun Facts : a well-known illegal drug, amphetamine, is actually 1-phenyl-2-propanamine.
Physical Properties
Boiling Point
With N-C bonding, it has higher boiling point than other unpolar organic substances, but still lower than other O-C-bonding polar ones such as Carboxylic acid or Alcoho.
Solubility
With its polar, it is soluble with water, except only aromatic amine. As usual, the bigger of molecule, the lower solubility.
Base of Amine
Since Nitrogen has 5 electrons and 3 of them are used for 3 single bonds, there are 2 of them left. Those 2 can be used to donate to Hydrogen ion (Proton.) Thus, Amine is base.
Reaction
1. Amide can be yield by reduction of Nitro.
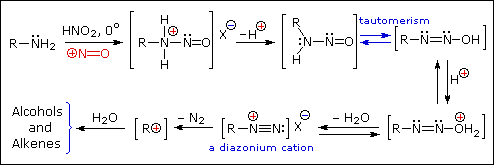
2. Reaction with Nitrous acid.
- Primary
Example :
- Secondary
Example :
- Tertiary
You will get nothing! (Just a weak base reacting with weak acid.)
Reference : BCC M.5 Chemistry Book.
http://en.wikipedia.org/wiki/Amine
http://www2.chemistry.msu.edu
http://www.chemguide.co.uk
http://www.pharmgkb.org

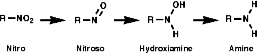
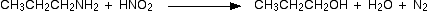
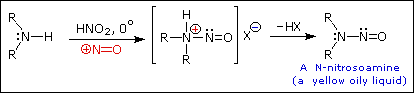
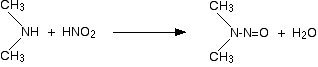
No comments:
Post a Comment