1. Polymerization of Styrene
We can have Polysterene (a kind of foam) by using continuously many Sterenes. Sterene, or Vinyl Benzene, is Benzene that has been replaced one of its Hydrogen with C2H2. So its formula is C6H5CH=CH2 as shown below.
Now, when we let Sterenes do a reaction at their C2H2 (because around their hexagon, they are aromatics, which are hard to do any reactions.) by destroying a pi bond in each of Sterenes in the free radical way so that unbonded electrons after the free radical will be used to be a sigma bond with other similar Sterenes.
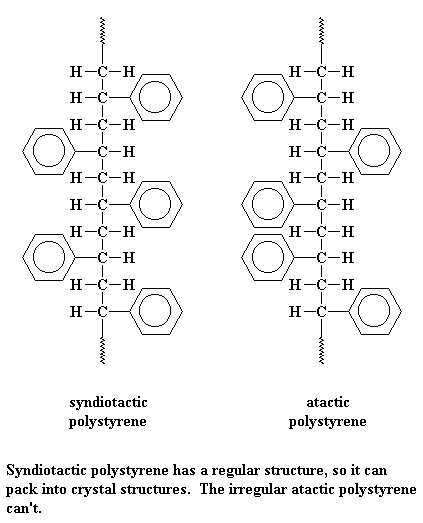
Bit of knowledge >>> Polysterene we get do not always need to have the aromatic groups (the hexagons you see) at the same side. For example: below are some of unneccessary ones.
References :
http://en.wikipedia.org/wiki/Styrene
http://en.wikipedia.org/wiki/Polystyrene
http://www.pslc.ws/mactest/styrene.htm


No comments:
Post a Comment