Hydrocarbon is an organic substance containing only carbon (C) and hydrogen (H). Alkanes is the hydrocarbon that every covalent bond is a single bond.
If it is a chain, this makes the number of H = 2 times of the number of C +2 , or written C(n)H(2n+2).
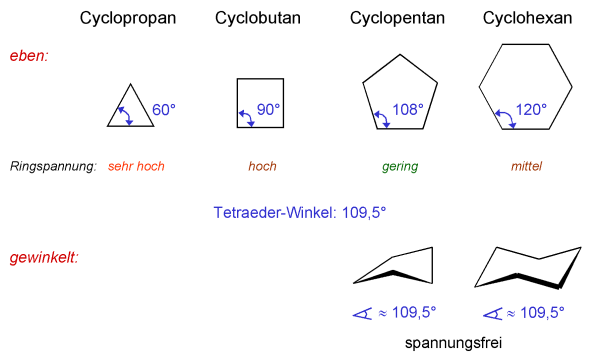
If it is a cycle (called cycloalkanes), this makes the number of H = 2 times of the number of C , or written C(n)H(2n).
Here is a few examples and their names.
http://www.u-helmich.de/che/12/03-oc/klassen/alkane/alkane01.jpg
http://www2.chemie.uni-erlangen.de/projects/vsc/chemie-mediziner-neu/kohlenwasserstoffe/bilder/cycloalkane.gif
Name
There are common name and IUPAC name. Common name is used for small and simple alkanes. However, IUPAC one is formal, official, and need to be used for complicated alkanes. We will know IUPAC name only since common name can be found every where while IUPAC should be found and used in HERE.
http://en.wikipedia.org/wiki/IUPAC_nomenclature_of_organic_chemistry
| Number of carbons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 20 | 30 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prefix | Meth | Eth | Prop | But | Pent | Hex | Hept | Oct | Non | Dec | Undec | Dodec | Tridec | Tetradec | Pentadec | Eicos | Triacont |
Now we know methane = CH4, Dodecane = C12H26.
1. Find the longest chain or cycle of carbon. Other alkyls (alkanes connectting) and atom are now considered as a brunch from chain/cycle.
2. Number orderly that longest one. Now we know what carbon we are talking about by the number.
3. However, there are somethimes many ways to number and find lostlest one. Use the one that the brunch appears as fast as possible when we numbered.
Ex. If 6-carbon chain with two methyls each at carbon 3 and 5 from left to right. We must numder from right to left since this makes the brunch appears at second carbon (from right), not third (from left).
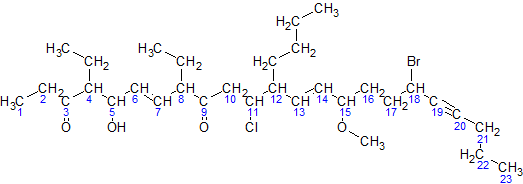
4. We need to call all the brunches in alphabet order with number to indicate their positions. For example, for picture above, brunched are 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricos-6,13-dien-19-yne-3,9-dione. (Name of brunch are difficult, well, just need to remember...)
5. then end with longest chain/cycle. For example, 23 =
Physical Properties
Table of alkanes
| Alkane | Formula | Boiling point [°C] | Melting point [°C] | Density [g·cm3] (at 20°C) |
| Methane | CH4 | -162 | -183 | gas |
| Ethane | C2H6 | -89 | -182 | gas |
| Propane | C3H8 | -42 | -188 | gas |
| Butane | C4H10 | 0 | -138 | gas |
| Pentane | C5H12 | 36 | -130 | 0.626(liquid) |
| Hexane | C6H14 | 69 | -95 | 0.659(liquid) |
| Heptane | C7H16 | 98 | -91 | 0.684(liquid) |
| Octane | C8H18 | 126 | -57 | 0.703(liquid) |
| Nonane | C9H20 | 151 | -54 | 0.718(liquid) |
| Decane | C10H22 | 174 | -30 | 0.730(liquid) |
| Undecane | C11H24 | 196 | -26 | 0.740(liquid) |
| Dodecane | C12H26 | 216 | -10 | 0.749(liquid) |
| Icosane | C20H42 | 343 | 37 | solid |
| Triacontane | C30H62 | 450 | 66 | solid |
| Tetracontane | C40H82 | 525 | 82 | solid |
| Pentacontane | C50H102 | 575 | 91 | solid |
Density
Bigger Molecule, higher density.
Melting and boiling points
Both are higher when the molecule are bigger. This is because Alkane has no polar, so the bond between molecule are London, which depends on mass.
Melting (blue) and boiling (pink) points of the first 14 n-alkanes in °C
http://en.wikipedia.org/wiki/Alkane
Polarity
As alkane have no polar or charge, so it is not solutable in water for sure.
Reaction
- Alkanes can be "cracked" into smaller pieces by add hydrogen and catalyst (which fixes the hydrogen not to move so it can bump... or do the reaction easier.)
- Hydrogen in alkanes can be replaced by other halogen atom.
- Alkanes can be made by add Hydrogen to alkenes or alkynes.
http://www.nature.com/nature/journal/v455/n7211/images/nature07369-f1.2.jpg
Same for cycloalkanes.
Reference : BCC M.5 Chemistry Book.



No comments:
Post a Comment