Alkene is similar to alkane. The only one difference is one of the bond between carbons are double, not single. This makes the number of Hydrogen atom twice of number of carbons. (CnH2n). Below is the alkene, with 6 carbon, called Hexene.
http://www.green-planet-solar-energy.com/alkene.html
However, as an example shown above, the position of double bond can be at many possible places. This gives the isomerism of alkene depending on double bond, which is important when we are learning how to call its name.
IUPAC Name
The difference of alkene and alkane is
- when we are finding the longest carbon chain, the chain we choose MUST contain the double bond.
- when we are numbering in order the carbon in the chain, the number we give to carbons with double bond must be as low as possible, regardless to any other brunches.
- The last word "alkene" such as "Hexene" must also tell where the double bond is. E.g. Hex-3-ene tells that the double bond is at third carbon. (see picture above.)
- When we look at the double bond, two carbons here are connected with other alkyls or hydrogens. Some maybe big or some maybe small. If the bigger ones are at the same side, use prefix "cis". If they are opposite, use "tran".
http://www.elmhurst.edu/~chm/vchembook/209cistrans.html
Reference : BCC M.5 Chemistry Book.
Sunday, November 21, 2010
Sunday, November 14, 2010
Organic Chemistry : Alkane
Alkanes & Cycloalkanes
Hydrocarbon is an organic substance containing only carbon (C) and hydrogen (H). Alkanes is the hydrocarbon that every covalent bond is a single bond.
If it is a chain, this makes the number of H = 2 times of the number of C +2 , or written C(n)H(2n+2).
If it is a cycle (called cycloalkanes), this makes the number of H = 2 times of the number of C , or written C(n)H(2n).
Here is a few examples and their names.
http://www.u-helmich.de/che/12/03-oc/klassen/alkane/alkane01.jpg
http://www2.chemie.uni-erlangen.de/projects/vsc/chemie-mediziner-neu/kohlenwasserstoffe/bilder/cycloalkane.gif
Name
There are common name and IUPAC name. Common name is used for small and simple alkanes. However, IUPAC one is formal, official, and need to be used for complicated alkanes. We will know IUPAC name only since common name can be found every where while IUPAC should be found and used in HERE.
http://en.wikipedia.org/wiki/IUPAC_nomenclature_of_organic_chemistry
Now we know methane = CH4, Dodecane = C12H26.
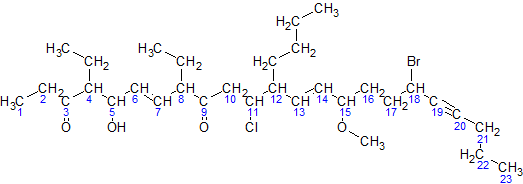
1. Find the longest chain or cycle of carbon. Other alkyls (alkanes connectting) and atom are now considered as a brunch from chain/cycle.
2. Number orderly that longest one. Now we know what carbon we are talking about by the number.
3. However, there are somethimes many ways to number and find lostlest one. Use the one that the brunch appears as fast as possible when we numbered.
Ex. If 6-carbon chain with two methyls each at carbon 3 and 5 from left to right. We must numder from right to left since this makes the brunch appears at second carbon (from right), not third (from left).
4. We need to call all the brunches in alphabet order with number to indicate their positions. For example, for picture above, brunched are 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricos-6,13-dien-19-yne-3,9-dione. (Name of brunch are difficult, well, just need to remember...)
5. then end with longest chain/cycle. For example, 23 =
Physical Properties
Density
Bigger Molecule, higher density.
Melting and boiling points
Both are higher when the molecule are bigger. This is because Alkane has no polar, so the bond between molecule are London, which depends on mass.
Melting (blue) and boiling (pink) points of the first 14 n-alkanes in °C
http://en.wikipedia.org/wiki/Alkane
Polarity
As alkane have no polar or charge, so it is not solutable in water for sure.
Reaction
- Alkanes can be "cracked" into smaller pieces by add hydrogen and catalyst (which fixes the hydrogen not to move so it can bump... or do the reaction easier.)
- Hydrogen in alkanes can be replaced by other halogen atom.
- Alkanes can be made by add Hydrogen to alkenes or alkynes.
http://www.nature.com/nature/journal/v455/n7211/images/nature07369-f1.2.jpg
Same for cycloalkanes.
Reference : BCC M.5 Chemistry Book.
Hydrocarbon is an organic substance containing only carbon (C) and hydrogen (H). Alkanes is the hydrocarbon that every covalent bond is a single bond.
If it is a chain, this makes the number of H = 2 times of the number of C +2 , or written C(n)H(2n+2).
If it is a cycle (called cycloalkanes), this makes the number of H = 2 times of the number of C , or written C(n)H(2n).
Here is a few examples and their names.
http://www.u-helmich.de/che/12/03-oc/klassen/alkane/alkane01.jpg
http://www2.chemie.uni-erlangen.de/projects/vsc/chemie-mediziner-neu/kohlenwasserstoffe/bilder/cycloalkane.gif
Name
There are common name and IUPAC name. Common name is used for small and simple alkanes. However, IUPAC one is formal, official, and need to be used for complicated alkanes. We will know IUPAC name only since common name can be found every where while IUPAC should be found and used in HERE.
http://en.wikipedia.org/wiki/IUPAC_nomenclature_of_organic_chemistry
| Number of carbons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 20 | 30 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prefix | Meth | Eth | Prop | But | Pent | Hex | Hept | Oct | Non | Dec | Undec | Dodec | Tridec | Tetradec | Pentadec | Eicos | Triacont |
Now we know methane = CH4, Dodecane = C12H26.
1. Find the longest chain or cycle of carbon. Other alkyls (alkanes connectting) and atom are now considered as a brunch from chain/cycle.
2. Number orderly that longest one. Now we know what carbon we are talking about by the number.
3. However, there are somethimes many ways to number and find lostlest one. Use the one that the brunch appears as fast as possible when we numbered.
Ex. If 6-carbon chain with two methyls each at carbon 3 and 5 from left to right. We must numder from right to left since this makes the brunch appears at second carbon (from right), not third (from left).
4. We need to call all the brunches in alphabet order with number to indicate their positions. For example, for picture above, brunched are 18-bromo-12-butyl-11-chloro-4,8-diethyl-5-hydroxy-15-methoxytricos-6,13-dien-19-yne-3,9-dione. (Name of brunch are difficult, well, just need to remember...)
5. then end with longest chain/cycle. For example, 23 =
Physical Properties
Table of alkanes
| Alkane | Formula | Boiling point [°C] | Melting point [°C] | Density [g·cm3] (at 20°C) |
| Methane | CH4 | -162 | -183 | gas |
| Ethane | C2H6 | -89 | -182 | gas |
| Propane | C3H8 | -42 | -188 | gas |
| Butane | C4H10 | 0 | -138 | gas |
| Pentane | C5H12 | 36 | -130 | 0.626(liquid) |
| Hexane | C6H14 | 69 | -95 | 0.659(liquid) |
| Heptane | C7H16 | 98 | -91 | 0.684(liquid) |
| Octane | C8H18 | 126 | -57 | 0.703(liquid) |
| Nonane | C9H20 | 151 | -54 | 0.718(liquid) |
| Decane | C10H22 | 174 | -30 | 0.730(liquid) |
| Undecane | C11H24 | 196 | -26 | 0.740(liquid) |
| Dodecane | C12H26 | 216 | -10 | 0.749(liquid) |
| Icosane | C20H42 | 343 | 37 | solid |
| Triacontane | C30H62 | 450 | 66 | solid |
| Tetracontane | C40H82 | 525 | 82 | solid |
| Pentacontane | C50H102 | 575 | 91 | solid |
Density
Bigger Molecule, higher density.
Melting and boiling points
Both are higher when the molecule are bigger. This is because Alkane has no polar, so the bond between molecule are London, which depends on mass.
Melting (blue) and boiling (pink) points of the first 14 n-alkanes in °C
http://en.wikipedia.org/wiki/Alkane
Polarity
As alkane have no polar or charge, so it is not solutable in water for sure.
Reaction
- Alkanes can be "cracked" into smaller pieces by add hydrogen and catalyst (which fixes the hydrogen not to move so it can bump... or do the reaction easier.)
- Hydrogen in alkanes can be replaced by other halogen atom.
- Alkanes can be made by add Hydrogen to alkenes or alkynes.
http://www.nature.com/nature/journal/v455/n7211/images/nature07369-f1.2.jpg
Same for cycloalkanes.
Reference : BCC M.5 Chemistry Book.
Thursday, November 11, 2010
Organic Chemistry : Introduction 2
Organic Reaction
In this topic, we will study about chemical reaction mechanism, so we will understand and be able to predict the result after the reaction of organic substance.
Bond forming
1. Each of the atom use one electron to make one covalent compound.
2. One of the atom use two electron to make one covalent compound.
Bond Cleavage
1. Homolytic - Each atom gets one electron resulting "Free Radical"
2. Heterolytic - When one atom has its EN more than another's, two electrons go to that atom, resulting one cation and one anion.
Stability of Intermediate
Carbon can be bonded with hydrogen or alkyl (commonly written as "R" - an alkane with one hydrogen taken away... to bond with this carbon instead.) Remember than alkyl has carbons, whose EN is more than hydrogen's, making it wants to give electrons to others since there are many electrons around carbons.
This concludes that the more alkyls carbon connects to, the more electrons likely to go into that carbon atom.
1. Carbon Radical = Carbon that lost 1 electron from 8 to 7. >> It needs 1 more electron>> want R = more R bonded with, more stability.
2. Carbonbocation (+) = Carbon that lost 2 electron from 8 to 6. >> It needs 2 more electrons >> want R = more R bonded with, more stability.
3. Carbonion (-) = Carbon that has full 8 electrons. >> It does not need anymore electrons >> hate R = more R bonded with, less stability.
Inductive Effect
All atoms do not have the same EN. Some are high while some are low. Also some atoms mat be an ion. This makes electrons likely to be found in different possibility in each point.
- If the electrons are dense or have - ion, it donates electron. For example, Alkyl (with the same reasons above.)
- If the atom have high EN valve, it withdraws electrons. For example, -O-H (Oxygen wants an electron.)
This can affect
1. Polarity. Electrons might flow some ways more often or not.
2. Stability & Reaction. Molacule with somewhere lots of electrons coming close has less stability - harder to do chemical reaction.
3. Acid. The less stability of bond connecting hydrogen, the more hydrogen flow away, therefore stronger acid.
4. Base. The higher density of the electrons around the atom, the more - charge there is, the more protons that want come stick with, therefore stronger base.
Resonance
Resonance is the state that there is an odd number of pi-electron pairs, running all way around molecule cycles or always switching between some different atoms. Rssonance makes molecule more stable.
Reference : BCC M.5 Chemistry Book.
In this topic, we will study about chemical reaction mechanism, so we will understand and be able to predict the result after the reaction of organic substance.
Bond forming
1. Each of the atom use one electron to make one covalent compound.
2. One of the atom use two electron to make one covalent compound.
Bond Cleavage
1. Homolytic - Each atom gets one electron resulting "Free Radical"
2. Heterolytic - When one atom has its EN more than another's, two electrons go to that atom, resulting one cation and one anion.
Stability of Intermediate
Carbon can be bonded with hydrogen or alkyl (commonly written as "R" - an alkane with one hydrogen taken away... to bond with this carbon instead.) Remember than alkyl has carbons, whose EN is more than hydrogen's, making it wants to give electrons to others since there are many electrons around carbons.
This concludes that the more alkyls carbon connects to, the more electrons likely to go into that carbon atom.
1. Carbon Radical = Carbon that lost 1 electron from 8 to 7. >> It needs 1 more electron>> want R = more R bonded with, more stability.
2. Carbonbocation (+) = Carbon that lost 2 electron from 8 to 6. >> It needs 2 more electrons >> want R = more R bonded with, more stability.
3. Carbonion (-) = Carbon that has full 8 electrons. >> It does not need anymore electrons >> hate R = more R bonded with, less stability.
Inductive Effect
All atoms do not have the same EN. Some are high while some are low. Also some atoms mat be an ion. This makes electrons likely to be found in different possibility in each point.
- If the electrons are dense or have - ion, it donates electron. For example, Alkyl (with the same reasons above.)
- If the atom have high EN valve, it withdraws electrons. For example, -O-H (Oxygen wants an electron.)
This can affect
1. Polarity. Electrons might flow some ways more often or not.
2. Stability & Reaction. Molacule with somewhere lots of electrons coming close has less stability - harder to do chemical reaction.
3. Acid. The less stability of bond connecting hydrogen, the more hydrogen flow away, therefore stronger acid.
4. Base. The higher density of the electrons around the atom, the more - charge there is, the more protons that want come stick with, therefore stronger base.
Resonance
Resonance is the state that there is an odd number of pi-electron pairs, running all way around molecule cycles or always switching between some different atoms. Rssonance makes molecule more stable.
Reference : BCC M.5 Chemistry Book.
Tuesday, November 2, 2010
Organic Chemistry : Introduction 1
Isomerism
Isomer molecules are molecules that all have the same number and exact types of atoms, but have different way of forming in molecules.
1. Structural Isomer
The position or bonding is different. Thus, it is easy to notice.
2. Stereo Isomer
Same bonding and position, but in the different directed angles such as mirror to another (called Optical.)
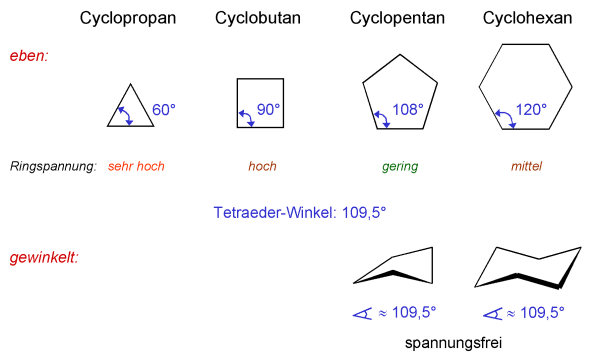
Types of Hydrocarbons
according to bondings
1. saturated : all bonds are sigma.
2. unsaturated : some bonds are pi.
according to structures
1. Aliphatic Hydrocarbon : uncyclic carbon chain.
2. Alicyclic Hydrocarbon : cyclic carbon chain.
3. Aromatic Hydrocarbon : cyclic carbon chain with Resonance.
4. Heterocyclic : cyclic carbon chain with some other atoms in the cycle.
according to functional groups
We can classify them in many many many .... manyyyyy functional groups.
Reference : BCC M.5 Chemistry Book.
Monday, September 20, 2010
Ion Equilibrium of Weak Electrolyte Solution (กรด-เบส) Contents
Table of Contents
0.1 Solubility
0.2 Rate of Reaction
0.3 Chemical Equilibrium
0.4 Introduction
1.1 นิยามของกรดเบส
1.2 คู่กรด-คู่เบส
1.3 ความแรงของกรด-เบส
การพิจารณาความแรงโดยดูจากโครงสร้าง
1.4 ตัวอย่างและประเภทของกรดและเบส
2.1 pH & pOH
2.2 pH Indicators
การเปลี่ยนสีของ Indicators
หลักการทำงาน
ค่า Ka ของ Indicator
ช่วง pH กับ Ka
3.1 Ka & Kb
3.2 การหา [H3O+] และ [OH-] จากสมดุลเคมี
3.3 Water & Hydrolysis
Kw of Water
Ka และ Kb ของคู่กรดเบส
Hydrolysis
3.4 Polyprotic
Questions ท้ายบท
4.1 ปฏิกิริยาระหว่างกรดเบส
เกลือ (Salts)
4.2 Buffer Solution
การพิจารณาเป็น Buffer
การผสมสารละลาย Buffer
4.3 Titration
Questions ท้ายบท What is in this blog?
Sunday, September 19, 2010
Ion Equilibrium of Weak Electrolyte Solution (กรด-เบส) Chapter 4
4. Multi - Acids & Bases
ในบทที่ผ่านๆมา เราจะกล่าวถึงกรดหรือเบสแต่ละตัวเมื่อละลายน้ำ แต่ในบทนี้จะพิจารณาเมื่อมีทั้งกรดและ/หรือเบสหลายๆชนิดพร้อมๆกัน ซึ่งอาจจะทำปฏิกิริยากัน เกิดเป็นสมดุลหลายๆสมดุลรบกวนกันไปมา หรืออาจไม่เกี่ยวข้องกันก็ได้
หลักในการมอง
ในบทเรียนทั่วๆไปอาจมีการจำแนกลักษณะปฏิกิริยา การผสมกัน เป็นประเภทหลายๆแบบ แต่ในที่นี้เราจะไม่มองเช่นนั้น แต่มองเป็น Animation ที่เกิดขึ้นตลอดเวลา โดยอธิบายจากสมดุล
1. สารประเภทที่ละลายน้ำหรือกรดแก่เบสแก่ ให้มองเป็ฺนสารหลังแตกตัวละลายอยู่เสมอ
2. เมื่อเรารุ้ทุกอย่างที่ละลายในน้ำ นำมาคิดต่อในสมดุลทุกอย่างที่จำเป็นต้องใช้ (สารบางอย่างอาจไม่ทำปฏิกิริยาหรือสมดุลไรต่อ ก็ตัดทิ้งไปได้)
3. ภายหลังเลื่อนปฏิกิริยาจนได้จุดสมดุล จึงสรุป
Ion Equilibrium of Weak Electrolyte Solution (กรด-เบส) Chapter 3
3. Dissociation of Acids and Bases & Hydrolysis
http://video.google.com/videoplay?docid=-7152825795445932495#
http://www.youtube.com/watch?v=AWaqpbDPlQE
http://video.google.com/videoplay?docid=-7152825795445932495#
http://www.youtube.com/watch?v=AWaqpbDPlQE
บทนี้เป็นบทหลักในการคำนวณหา [H+] (หรือ [H3O+]) และ [OH-] เพื่อรายงานค่าเป็น pH ให้ได้
3.1 Ka & Kb
เมื่อเรานำกรดอ่อนชนิดหนึ่งไปละลายน้ำ
HA + H2O <---> A- + H3O+
จะเกิดสมดุลขึ้น มีค่าคงที่สมดุลคือ [A-][H3O+] / [HA][H2O]
แต่เนื่องจากในปฏิกิริยา มี [H2O] อยู่เยอะมาก (ลองคิดดูโดยสมมติน้ำหนาแน่น 1kg/L และ 1molH2O = 18g จะได้ [H2O]=55.55 M) แม้สมดุลจะเป็นอย่างไร [H2O] จึงเปลี่ยนแปลงน้อยมาก เราจึงถือว่า [H2O] เป็นค่าคงตัว
ดังนั้นจึงนิยามใหม่เป็น
Ion Equilibrium of Weak Electrolyte Solution (กรด-เบส) Chapter 2
2. pH & pH Indicators
เราทราบการแตกตัวของกรดและเบสแล้ว จะเห็นได้ว่าการแตกตัวนั้นมีความสัมพันธ์กับ H+ และ OH- อย่างมาก ในบทนี้เป็นการให้นิยามความหมายของ pH และรู้จักกับ pH Indicator ซึ่งจะใช้ในการรายงานความเป็นกรดเป็นเบสในบทถัดๆไปได้
2.1 pH & pOH
ในสารละลายกรดหรือเบส เราจะพบ H+ (หรือ H3O+ ก็ได้ เป็็นอย่างเดียวกัน) และ OH- ละลายอยู่ในปริมาณหนึ่ง ปริมาณนี้มักเป็นปริมาณที่น้อย เช่น มี H+ ละลายอยู่ 1.4*10^-4 M (molar) เป็นต้น หากต้องรายงานผลเป็นเลขยกกำลังติดลบเช่นนี้ตลอดเวลาจะเป็นการยุ่งยาก จึงมีการนิยาม pH (Power of Hydrogen) ว่า
Ion Equilibrium of Weak Electrolyte Solution (กรด-เบส) Chapter 1
1. Acids and Bases
ในบทนี้จะเป็นการทำความรู้จักกับกรดเบสก่อนว่าคืออะไร ความแรงหมายถึงอะไร ดูได้อย่างไร พร้อมตัวอย่างและการแบ่งประเภท
1.1 นิยามของกรดเบส
http://www.youtube.com/watch?v=tNtdXAMsyXw&feature=related
http://www.youtube.com/watch?v=jLcoIRUlz20&feature=related
http://www.youtube.com/watch?v=tNtdXAMsyXw&feature=related
http://www.youtube.com/watch?v=jLcoIRUlz20&feature=related
ข้อจำกัด : สารต้องละลายน้ำ และหากไม่มี H หรือ OH ในสารก็จะไม่ได้สามารถบอกได้
- เบรินเสตด-ลาวรี มองการนิยามกรด-เบส จากสมดุล
HA + B <---> A- + BH+
โดยให้นิยามว่า
Ion Equilibrium of Weak Electrolyte Solution (กรด-เบส) Chapter 0
0. Basics & Introduction
บทนี้จะเป็นการปูพื้นฐานในเรื่องที่จำเป็นก่อนเข้าสู่เนื้อหาจริง
การพิจารณาการละลายในที่นี้ จะพิจารณาเฉพาะสารประกอบไอออนิกที่ละลายในน้ำ สิ่งที่ควรทราบคือ สารประกอบจะละลายน้ำ ถ้า
Subscribe to:
Comments (Atom)